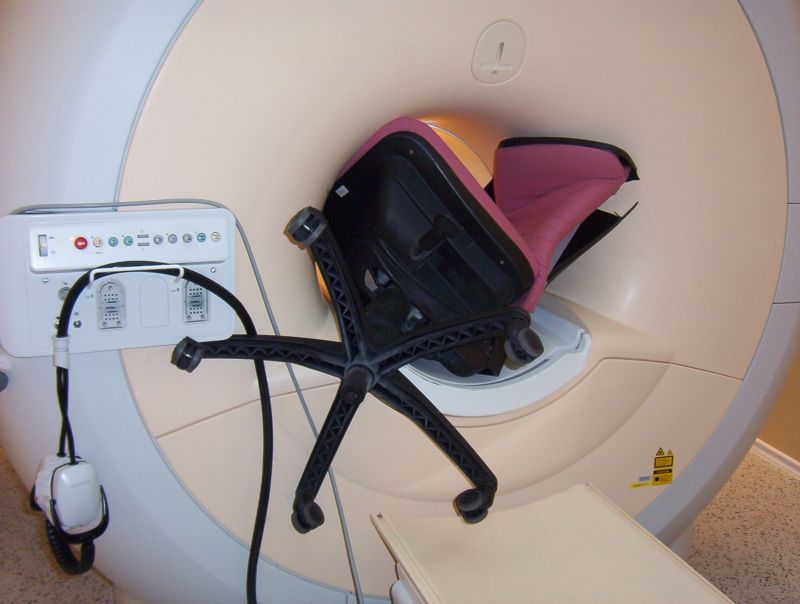
An office chair was in the wrong place - at ANY time!

MRI Screening Form for Patients
Click Here to Download The Forms in Adobe's PDF Format
! This file requires Adobe's Acrobat Reader in order to View and Print. If you need Acrobat Reader, please click the button below.

© by Shellock R & D Services, Inc. and Frank G. Shellock, Ph.D. All rights reserved.